Sodium Bromide And Chlorine Balanced Equation . when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. all atoms are now balanced and the whole equation is fully balanced: Cl 2 + 2 nabr = 2 nacl + br 2. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. Balancing step by step using. in this video we'll balance the equation nabr + cl2 = nacl + br2 and.
from www.chegg.com
Balancing step by step using. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. in this video we'll balance the equation nabr + cl2 = nacl + br2 and. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. all atoms are now balanced and the whole equation is fully balanced: Cl 2 + 2 nabr = 2 nacl + br 2. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine.
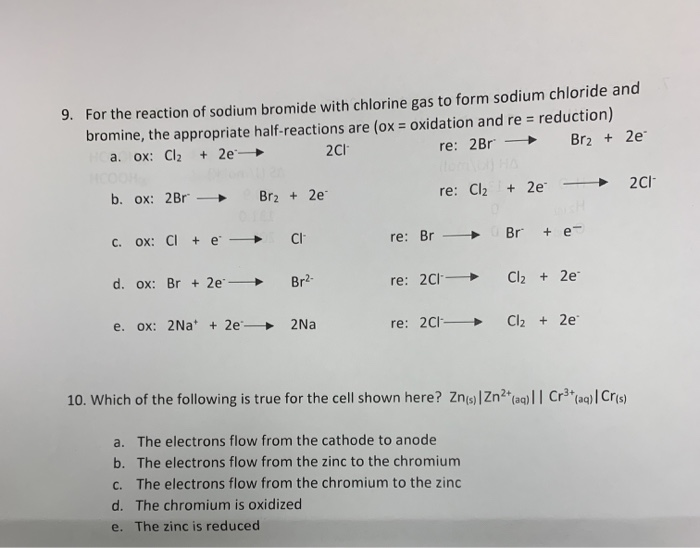
Solved 9. For the reaction of sodium bromide with chlorine
Sodium Bromide And Chlorine Balanced Equation in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. all atoms are now balanced and the whole equation is fully balanced: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in this video we'll balance the equation nabr + cl2 = nacl + br2 and. Cl 2 + 2 nabr = 2 nacl + br 2. Balancing step by step using. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Sodium Bromide And Chlorine Balanced Equation in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Balancing step. Sodium Bromide And Chlorine Balanced Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Sodium Bromide And Chlorine Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. in this video we'll balance the equation nabr + cl2 = nacl + br2 and. nabr + cl = br + nacl is. Sodium Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Sodium Bromide And Chlorine Balanced Equation diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. nabr + cl. Sodium Bromide And Chlorine Balanced Equation.
From www.coursehero.com
Using the balanced equation below, how many grams of sodium bromide Sodium Bromide And Chlorine Balanced Equation when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along. Sodium Bromide And Chlorine Balanced Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Sodium Bromide And Chlorine Balanced Equation when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. in this video we'll balance the equation nabr + cl2 = nacl + br2 and. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another. Sodium Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Solid sodium bromide into bromine gas and solid Sodium Bromide And Chlorine Balanced Equation nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. Cl 2 + 2 nabr = 2 nacl + br 2. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another example of a chemical reaction, sodium metal reacts. Sodium Bromide And Chlorine Balanced Equation.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Sodium Bromide And Chlorine Balanced Equation in this video we'll balance the equation nabr + cl2 = nacl + br2 and. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. Balancing step by step using. Cl 2 + 2 nabr = 2 nacl + br 2. diatomic chlorine and sodium hydroxide (lye). Sodium Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Sodium Bromide And Chlorine Balanced Equation diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Cl 2 + 2 nabr = 2 nacl + br 2. all atoms are now balanced and the whole equation is fully balanced: nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of. Sodium Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Sodium bromide reacts with calcium chloride to form sodium Sodium Bromide And Chlorine Balanced Equation diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. all atoms are now balanced and the whole equation is fully balanced: in this video we'll balance the equation nabr + cl2 = nacl + br2 and. nabr + cl = br + nacl is a single displacement (substitution). Sodium Bromide And Chlorine Balanced Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Sodium Bromide And Chlorine Balanced Equation Balancing step by step using. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. in this video we'll balance the equation nabr + cl2 = nacl + br2 and. diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,.. Sodium Bromide And Chlorine Balanced Equation.
From www.youtube.com
Type of Reaction for NaBr + Cl2 = NaCl + Br2 YouTube Sodium Bromide And Chlorine Balanced Equation nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Cl 2 + 2 nabr = 2 nacl + br 2. in another example of a chemical reaction, sodium metal reacts. Sodium Bromide And Chlorine Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for NaBr + Cl2 = NaCl + Br2 YouTube Sodium Bromide And Chlorine Balanced Equation when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. in this video we'll balance the equation nabr + cl2 = nacl + br2 and. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium. Sodium Bromide And Chlorine Balanced Equation.
From www.youtube.com
How to Balance NaBr + Cl2 = NaCl + Br2 (Sodium Bromide + Chlorine gas Sodium Bromide And Chlorine Balanced Equation Balancing step by step using. all atoms are now balanced and the whole equation is fully balanced: in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. To balance. Sodium Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Which products would form if chlorine gas was bubbled through a Sodium Bromide And Chlorine Balanced Equation when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. nabr + cl = br + nacl is a single displacement (substitution) reaction where. Sodium Bromide And Chlorine Balanced Equation.
From www.numerade.com
When chlorine gas is bubbled into a solution of sodium bromide, the Sodium Bromide And Chlorine Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. Balancing. Sodium Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVEDBromine can be prepared by adding chlorine to an aqueous Sodium Bromide And Chlorine Balanced Equation when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. Cl 2 + 2 nabr = 2 nacl + br 2. all atoms are. Sodium Bromide And Chlorine Balanced Equation.
From www.slideserve.com
PPT Double Replacement 1. Hydrogen sulfide is bubbled through a Sodium Bromide And Chlorine Balanced Equation Balancing step by step using. Cl 2 + 2 nabr = 2 nacl + br 2. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. nabr + cl. Sodium Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced molecular equation for the reaction indicated Sodium Bromide And Chlorine Balanced Equation nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. all atoms are now balanced and the whole equation is fully balanced: in this video we'll balance the. Sodium Bromide And Chlorine Balanced Equation.